.jpg)
FDA to Approve Innovative Medical Devices in Half the Time (MedScape)
FDA Launches Medical Device Innovation Initiative (FDA)
FDA Introduces Medical Device Innovation Initiative (HealthPointCapital)
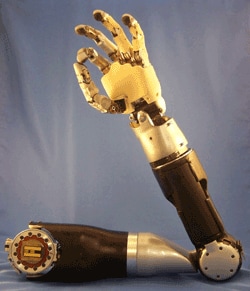
The FDA proposes the Innovation Pathway, a priority review program for new, breakthrough medical devices and announced that the first submission will be a brain-controlled, upper-extremity prosthetic that will serve as a pilot for the program. The FDA also announced plans to seek further public comment before the Pathway can be used more broadly. The FDA will host a public meeting on March 15th.
