
Answering the calls: 30 years of Himed history (Himed website)
As President of Himed, Ed Garofalo oversees a business that’s now a leading global provider of cutting-edge biomaterials and surface treatments for the dental and orthopedic industries. Yet thirty years ago, if you went looking for Ed, you’d find him working with metal coatings for engine turbines at a family-owned business in New York called Hitemco.
Of course, engine turbine parts and a hip implant don’t have much in common on paper, but it’s safe to say that the finish on both had better be unquestionably precise before they’re put to use.
From engine turbines to medical implants
In the late ‘80s, the medical implant industry looked very different than it does today. Medical advancements were coming at a respectable pace but a flexible, adaptable supply chain simply wasn’t in place the way it is now. That’s why Ed would get calls from members of the medical industry asking if Hitemco could provide finishes on orthopedic implants.
One major issue with implants back then was the cement used to bond the implants to bone. The cement would heat up, degrade or even crack the bone, and sometimes break off completely over time. Terrible scenarios, all of them, which often required additional corrective surgeries. The solution didn’t come in the form of better cement, but a different approach entirely.
That approach was to create a rough, beaded surface on a metal implant that was then pressure-fit into the bone. The body would naturally develop tissue around the implant, using the textured surface as a scaffold. Hitemco was uniquely suited to create those finishes on cobalt-chrome implants.
What’s hydroxyapatite, anyway?
Once members of the medical community got wind of Hitemco’s plasma spray coatings, Ed started getting more calls. Could Hitemco perhaps put hydroxyapatite (HA) on implants? Ed was pretty sure they could…if they knew what the heck hydroxyapatite was. Curious and inspired, Ed hit the phones (remember, Google didn’t exist back then). He searched for suppliers and gathered up any information he could. He soon learned that HA is a naturally occurring calcium phosphate which can be found, albeit in modified forms, in both human bones and teeth.
Unlike metals and alloys, Ed didn’t have a ready idea of where to acquire HA, so he put in the hours to figure it out. After all, this wasn’t the first time a client request came from out of left field—and it was far from being the last.

Back then, the market for this type of calcium phosphate wasn’t especially large—and consistent supply of the material was largely unavailable. One day Ed came across the NYU College of Dentistry and their Calcium Phosphate Research Lab. If anyone knew about HA, it would be the researchers at a lab that was practically named for the stuff.
Finding exactly the right people
On a visit to the NYU lab, Ed had a fortuitous meeting with Dr. Raquel Le Geros. She was at the forefront of research around the use of hydroxyapatite in dental applications and traveled the world giving talks on the subject. Few people knew more about HA than Dr. Le Geros.
Her husband, John, was also a medical researcher and after several discussions between the Le Geroses, Ed, and the founder of Hitemco, Richard Barnard, the four realized the singular opportunity they had on their hands. Hitemco knew surface coatings. The Le Geroses knew HA. The medical community was in great need of HA coatings and products. Teaming up just made sense.
March 25, 1991: Himed is born
With the Le Geroses providing their expertise, Ed shifted part of his time away from Hitemco to develop Hitemco Medical Applications (doing business as Himed). He was joined by biomaterials scientist and engineer Jinlong Wang, who would later become Himed’s VP of Biomaterials. In the beginning, Himed managed just a few sales, adding up to about $6,000 the first year. While sales may have been slow, however, Himed was quite busy.
Convinced there was a way to make a plasma-sprayable HA powder, Ed and a small team led by Jinlong Wang spent the next four years researching—until their hypothesis proved correct. The substance and techniques the team developed are now used around the world and on millions of medical implants.
A fortunate discovery
While searching for the solution to a plasma-sprayable HA, Ed had a eureka moment. (You could even call it his “a-HA” moment.) Creating textured surfaces on implants was (and is) incredibly important.
Most grit blasting of implant surfaces at the time was done with aluminum oxide. While the substance is very hard and great for texturizing, there’s simply no way to get all the residue out of the implant’s surface after blasting. Brushing, ultrasound, nothing will do the trick…
And that’s a problem. If implanted into the body, that residue could create an inflammatory reaction, compromising the implant. One solution was to coat the surface to keep the residue from contact within the body. However, the grit particles from the aluminum oxide embedded beneath the coating could become what’s known as a “stress riser” which concentrates pressure, thereby weakening the mechanical strength of the whole device.
So Ed found a better solution. He realized that if Himed could make a harder form of calcium phosphate, it could be used as an abrasive for implants. At the time, there wasn’t a name for it, but now it’s known as resorbable blast medium (RBM) or soluble blast medium (SBM).
Even if this blast medium left behind trace amounts of residue, it theoretically wouldn’t matter, thanks to the mineral’s excellent biocompatibility.
Just hard enough
There is one notable limitation to abrading with calcium phosphate grit—even when it is manufactured to be as hard as possible it cannot exceed a 5 on the Mohs’ scale. Aluminum oxide? That rates near the top of the scale at a hardness of 9. So CaP couldn’t do much to the surface of stainless steel, and it would have zero effect on cobalt-chrome.
One material it could abrade, however, was titanium. And titanium, which also happens to be hypoallergenic, light weight, and possess a high tensile strength, seemed a logical material for the next generation of medical implants.
Ed reached out to implant manufacturers to tout the benefits of Himed’s new development: MCD apatitic abrasive. Titanium implants could now have their surfaces roughed with a soluble media, giving the bone something to grab onto, with none of the adverse side effect potentials from traditional blasting.
Taking it one step further, Himed researchers were also able to show that by using MCD apatitic abrasive just prior to the normal implant passivation step, the RBM residue would dissolve, creating a surface that was exceptionally compatible with the body.

Because titanium wasn’t the most prevalent material for implants back in the ‘90s, Ed didn’t initially receive huge interest from the implant manufacturing market. But one company did see the potential. They happened to be one of the larger implant manufacturers and once they were on board—and other companies saw the benefits—the adoption of this “resorbable blast media” (RBM) rapidly took off.
Doctors loved this new technique. Implant designs improved—as did patient outcomes. Today, RBM is the industry standard for texturizing titanium implants.
Risky business
By 1994, Ed had grown the tiny Himed team from a one-room operation at the back of Hitemco to a two-room operation. The atmospheric plasma-sprayed hydroxyapatite (APS HA) idea was now a reality, which made Himed one of the few companies possessing the ability.

Additionally, Himed’s soluble apatitic abrasive, and their corresponding blasting service using that grit, was taking off. For both the APS HA and the RBM developments, Ed had kept all of the R&D in-house. Guided by inquiries from potential clients and the team’s own creative problem solving, Himed had spent the better part of four years researching these breakthroughs—with little income stream and no guaranteed client interest once the products were developed.
It was a risky move, business-wise; a whole lot of time and resources invested in ideas that relied on possible developments in the implant industries, which may not pay off in the end.
But it did pay off. Himed is now the number one supplier of RBM globally and their plasma-sprayable HA powder and spraying capabilities are used on hundreds of thousands of implants year after year.
“I have a number of axioms, and one of them is, things happen to you and you make the most of them. So, while you’ve got things you’re planning, in the meantime you also want to be opportunistic when something you didn’t expect comes up.
— Ed Garofalo, Himed President
Not just better, but faster too
Fast-forward to 2004 and Himed’s customer base was expanding rapidly. But other suppliers were growing, too. Ed already knew Himed had excellent products. The next goal would be to deliver them faster, and with better customer service, than anyone else in the industry.
That led to a major site expansion for Himed: in addition to creating the space for resources to fulfill customer requests faster, the facility now housed on-site automation. Back when he was VP of Operations for Hitemco, Ed fueled the company’s leap into robotic spray systems, a move that cut job set-up time from a half day to a matter of minutes. The investment wasn’t small, but the payoff was worth it, allowing Hitemco to scale up the scope of their projects and expand capacity within months.
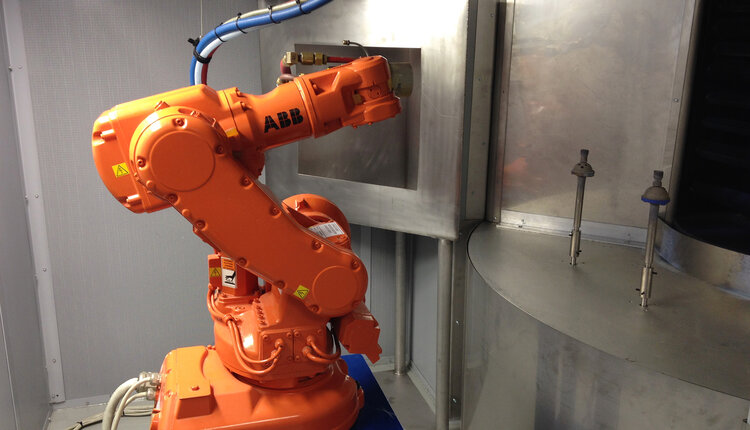
Applying the same logic, Himed invested heavily in automation for creating forms, plasma-spraying, surface treatments, and more. The result? Himed could now turn most customer orders around in rapid time, much quicker than the competition. Paired up with a more competitive price, and a customer service ethic carried over from the family-owned ethos of Hitemco, Himed was able to scale up dramatically.
Even better than cow teeth
By 2012, Himed had moved into a state-of-the-art 25,000-square foot facility adjacent to Hitemco with 5,000 square feet of laboratory clean rooms. The move only made the innovations come faster. Thanks to ongoing internal research leading to more and more discoveries, Himed decided to gather its growing variety of in-house implant surface treatments under the name MATRIX™.

The new expansion wasn’t just great for Himed’s ability to innovate, it also allowed Himed to improve their quality assurance. With enhanced analytical capacity, including new vision systems, many inspection processes could now be automated—improvements that led Himed to set its sights on seeking ISO and FDA recognitions.
All the while, customers continued to call Himed and ask, “Can you do this?” and the R&D team would eagerly figure out if there was a way to meet those needs. One customer needed a better way to test toothpaste, whiteners, and other dentifrices. The techniques for testing such products at that time included using cow or cadaver teeth. Unfortunately, those substrates have a high degree of variability which confounds the experimental results. Using sub-par materials, researchers were being denied the ability to gather valuable and reliable data.

This led to Himed developing gel-cast CaP solids in forms such as teeth that could be mounted in a position that mimicked the human mouth, allowing researchers to test the products in ways that would be found in real people. The efficacy of testing was greatly improved.
Other labs quickly saw the value in other substrates that would help with scientific research. Himed developed ways to supply everything from hydroxyapatite discs for direct experimentation, to CaP forms that could be machined into parts for implant elements. Using hydroxyapatite in implants was particularly intriguing as such parts could potentially become resorbable implant elements—with considerable patient benefits.
High purity, high performance, and lower cost
After such unparalleled successes in the dental implant market, Himed refocused its efforts on orthopedics. In 2015, Craig Rosenblum was hired as Himed’s Engineering Manager, and he immediately began to tackle different process development projects related to the MATRIX™ line of surface enhancements. Under his guidance, Himed officially launched a novel titanium coating process for hip implants that they had patented in 2013.
While other companies were already offering textured titanium coatings, they were doing so under expensive vacuum conditions. Himed’s process was unique in that it was performed in atmospheric conditions at a fraction of the cost—with zero reduction in the performance of the coating.

Himed’s process was named MATRIX Ti™. Used in combination with Himed’s MCD abrasive, implants undergoing this process received a commercially pure, porous surface ideally suited for bone regrowth. Medical implants coated with MATRIX Ti™ were approved by the FDA in 2016, and a CE mark for similar use in Europe was obtained in 2018.
The future will be in 3D
After making significant, industry-changing strides in coatings, CaP forms, and surface treatments, Himed wasn’t content to sit still. Looking to the future, they wondered what might be the next generation of medical implant innovations. What about resorbable implants created to the exact specifications of each patient
Himed sees that idea getting closer and closer to reality with advancements in 3D printing. Specialized CaP forms and mixes may soon be used as the printer’s “inks,” allowing surgeons to 3D print bone scaffolding directly: built to the exact specifications of the patient’s precise implant needs.
Himed aims to be a major contributor to these new solutions.
“I think the big change will be from cast or forged implants to 3D-printed versions that are made for each patient. Whatever your defect is, the implant is going to match it. With 3D printing, you can literally go from having a model where everybody gets the standardized implant, to a model where you get an implant, specific to you, at a lower cost.
— Ed Garofalo, Himed President
The promise of bespoke implants
Himed has been working for more than ten years on improving biocompatible scaffolding agents, preparing for the day that on-demand printing of implants is a reality. There’s no question that HA will play a key role in implant manufacturing via 3D printing, thanks to thirty years of strong scientific evidence for HA’s ability to support osseointegration, and broad acceptance of HA coatings in major global markets.
This technological shift will mean completely bespoke fabrication of implants on a patient-by-patient basis, yielding perfectly shaped implants that seamlessly integrate into the body. Lead times will virtually disappear and wasted material will be a thing of the past.
Doubled capacity and evolving surfaces
In 2019, with increasing demand, Craig and the engineering team doubled Himed’s production capacity of MCD apatitic abrasive by implementing two more industrial furnaces. That same year, Himed publicly launched an advanced application of its standard MATRIX MCD™ abrasive blast texturing process, naming the new iteration MATRIX Dual™.
With MATRIX Dual™, titanium implant surfaces are blasted to create smaller pores inside of larger pores. The result is a surface with exacting uniformity on even the most geometrically complex and smallest surfaces. For device manufacturers looking for the utmost control over a textured surface, MATRIX Dual™ offered resorbable blast texturizing with a much more complex surface morphology than single blast texturing.
A brand new CEO with a Himed lineage
In 2020, Dana Barnard joined Himed as CEO. Dana came to the company fresh off the sale of a tech company that had developed a unique cold-chain storage technology used in the emerging cell and gene therapy industry. He was no stranger to Himed’s dedicated focus on innovation.
In fact, he was no stranger to Himed at all. Nearly thirty years ago, Dana’s father, Richard, worked with Ed and the Le Geros team to form Himed. Dana had stayed connected to Himed over all those years, but was excited to work more closely with the company on further market expansions.
Dana could plainly see where the future of the orthopedic and dental implant markets was heading. Demand in the industry would continue to increase in response to the aging baby boomer population. That will drive innovation quickly, and Dana wanted to ensure that Himed would remain at the forefront of biomaterials innovation, offering novel solutions to those expanding markets and the patients they serve.

“I keep coming back to this idea that Himed’s been a global operation from the beginning, and has been exposed to a hugely varied demand. Himed has this massive brain and remarkable amount of experience that’s rare for a small company, because most small companies are regional. But we deal with the whole world, so we see many different applications of bioactive materials and surfaces, and that keeps us on the cutting edge of technical developments.
— Dana Barnard, Himed CEO
Looking forward to the next 30 years
Dana’s first challenge with the Himed team was ensuring the company could maintain production during the volatile first months of the COVID-19 pandemic (which it did). He then turned his attention to more actively sharing Himed’s remarkable story. New marketing and outreach efforts, including the publication of Himed’s first whitepaper about the additive finishing potentials of MATRIX MCD™, helped Himed’s outward-facing image start to look as impressive as what went on behind the scenes. Within only a few months of these efforts, Himed became much more visible.
Before, Himed had quietly grown through constant innovation and real-world results. But now, the usual word-of-mouth promotion was joined by a new group of start-ups and manufacturers—exciting new partners and clients who had found Himed on the web or in trade magazines.
Still answering the calls
It does seem improbable sometimes that one day in 1991, Ed and Jinlong started working out a way to spray HA coatings on medical implants, and thirty years later, Himed has changed the implant industry for the better. From one room at the back of a metal spray coatings company to a 25,000 square foot facility and a team of thirty-five, Himed has come a long way.
But one thing remains the same: the phone still rings. And while Ed might not always be the one taking the call, the conversation goes much the same. A customer asks, “Can you do this?” and more often than not, the Himed team answers with a resounding, “YES.”

