Click the to learn more
Greenlight.guru helps medical device companies bring new products to market faster while ensuring their regulatory compliance & mitigating risk. We built a completely new & innovative QMS software solution exclusively for medical device companies that’s actually practical for them to use.
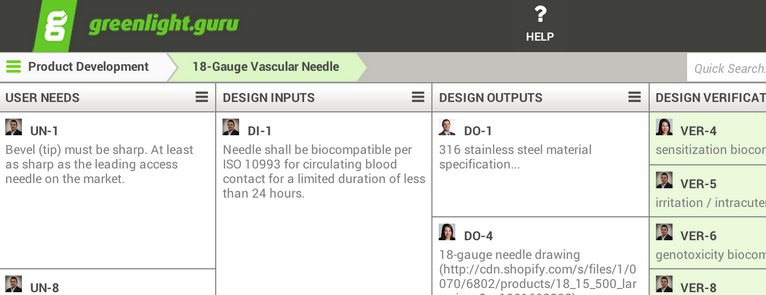
greenlight.guru provides medical device companies a Single Source of Truth by integrating all their document management, design control, electronic signatures, quality process, and training management activities together into one, dead simple to use software solution that takes hours to pick up, not months. This Single Source of Truth drastically improves team productivity and collaboration, ensures compliance by enforcing FDA best practices and greatly reduces the risk of being cited during an audit by providing a full FDA CFR Part 11 compliant audit trail.
Quotes / Testimonial
 Allan Katz, President & CEO of Long Island Technology Group: “Medical device companies must document Design Controls, yet most struggle with this. greenlight.guru makes this so much easier. Another HUGE headache for medical device companies is document management. And greenlight.guru takes care of this too.”
Allan Katz, President & CEO of Long Island Technology Group: “Medical device companies must document Design Controls, yet most struggle with this. greenlight.guru makes this so much easier. Another HUGE headache for medical device companies is document management. And greenlight.guru takes care of this too.”
 Evan Jones, Product Development Engineering at Long Island Technology Group: “This is my first time going through medical device product development. I’ve heard about all the challenges with Design Control. With greenlight.guru, I know what to do and when to do it. Documenting Design Controls is really easy with this software.”
Evan Jones, Product Development Engineering at Long Island Technology Group: “This is my first time going through medical device product development. I’ve heard about all the challenges with Design Control. With greenlight.guru, I know what to do and when to do it. Documenting Design Controls is really easy with this software.”
Click the to learn more

 Tiger Buford – retained recruiter dissecting orthopedics
Tiger Buford – retained recruiter dissecting orthopedics