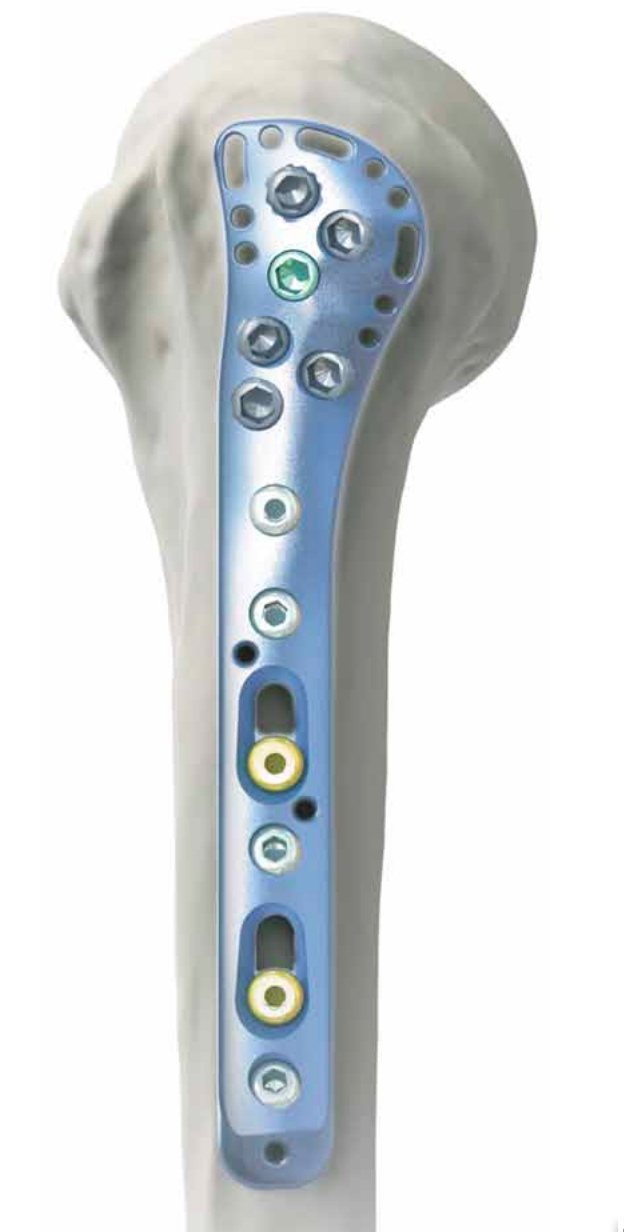
 Shoulder Options, Inc. is seeking to divest of its proximal humerus plate devices. The plating systems were designed for treatment of proximal humerus fractures and for rotator cuff repair, are 510(k) approved by the U.S. Food and Drug Administration (FDA), and have been granted utility and design patents by the U.S. Patent and Trademark Office (USPTO). The devices include the AFT™ Proximal Humerus Fracture Plate, the AFT™ Greater Tuberosity Fracture Plate, and the CRP™ Cuff Repair Plate.
Shoulder Options, Inc. is seeking to divest of its proximal humerus plate devices. The plating systems were designed for treatment of proximal humerus fractures and for rotator cuff repair, are 510(k) approved by the U.S. Food and Drug Administration (FDA), and have been granted utility and design patents by the U.S. Patent and Trademark Office (USPTO). The devices include the AFT™ Proximal Humerus Fracture Plate, the AFT™ Greater Tuberosity Fracture Plate, and the CRP™ Cuff Repair Plate.
“Shoulder Options has created innovative plating systems for anatomical repair of the proximal humerus,” commented T. Bradley Edwards, MD, of the Fondren Orthopedic Group in Houston, TX. Dr. Edwards, a member of the fracture plate design team, is recognized internationally as a leading author and speaker on orthopedic shoulder topics.
“Our devices were designed by a world-class team of orthopedic shoulder specialists,” said C. Scott Humphrey, MD, who is serving as the company’s interim CEO. “These plates have successful clinical track records, but our challenge as a small company has been achieving distribution on a larger scale. We hope to place our devices with a bigger company to make them accessible to more patients.”
Interested parties may visit the company’s website at http://www.shoulderoptions.com, or contact Dr. Humphrey at humphrey@shoulderoptions.com.
