ALTIOR TRAUMA ANNOUNCES FDA 510(K) CLEARANCE FOR THE ARTEMIS PROXIMAL FEMORAL NAIL SYSTEM (press release)
A FOURTH-GENERATION HIP FRACTURE NAIL MANUFACTURED USING A REVOLUTIONARY PROCESS
Altior Trauma, a GLW, Inc. company, (“Altior Trauma”) announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration for its Artemis Proximal Femoral Nail System (the “Artemis System”), a hip fracture nail. The Artemis System offers an advancement in hip fracture treatment that combines the strength of titanium with a unique blend of Solvay Zeniva® carbon fiber reinforced polyetheretherketone (“CF PEEK”), a radiolucent polymer. This proprietary process of combining materials allows for greater skeletal visualization and has the potential for the promotion of fracture healing.
“The Artemis System is truly a global product platform that delivers modern innovation. I am incredibly proud of our engineers and what they have accomplished in order to deliver a next generation hip fracture nail utilizing a patent pending advanced manufacturing process that significantly reduces costs,” said Vadim Gurevich, CEO of Altior Trauma. “The launch of Artemis, our first of many products, will announce to the world that we are here to improve hip fracture technology and care, while reducing the overall cost to the healthcare system.”
Addressing the growing socio-economic problems of treating hip fractures in elderly patients, the Artemis System is designed to solve a wide range of unmet surgical treatment needs for surgeons, OR staff, hospital administrators, and, most importantly, patients. Dr. H. Claude Sagi, orthopaedic traumatologist at University of Cincinnati Health, emphasized that, “Hip fracture fixation has lacked any significant innovation in the last 20+ years. The Artemis System has the potential to transform the hip fracture fixation market by providing a significant outcome-based design, reducing procedural complexity, and lowering the cost through innovative engineering technologies when compared to other hip fracture nails.”
“The radiolucency of the CF PEEK provides significantly clearer visualization during implantation which allows for a more satisfying procedure and an additional degree of informative postoperative x-rays,” said Dr. Steven S. Sands, orthopaedic trauma surgeon at Integris Health.
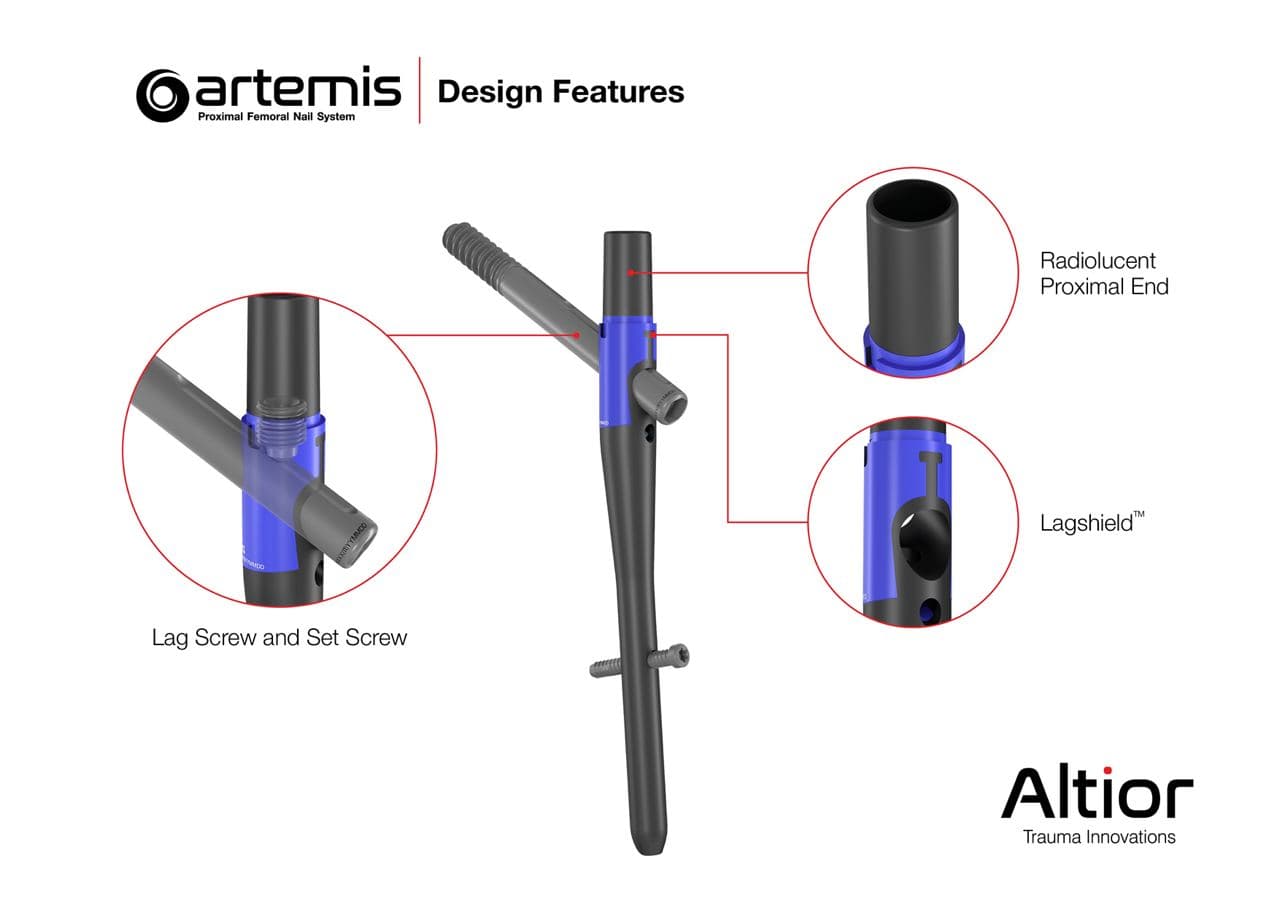
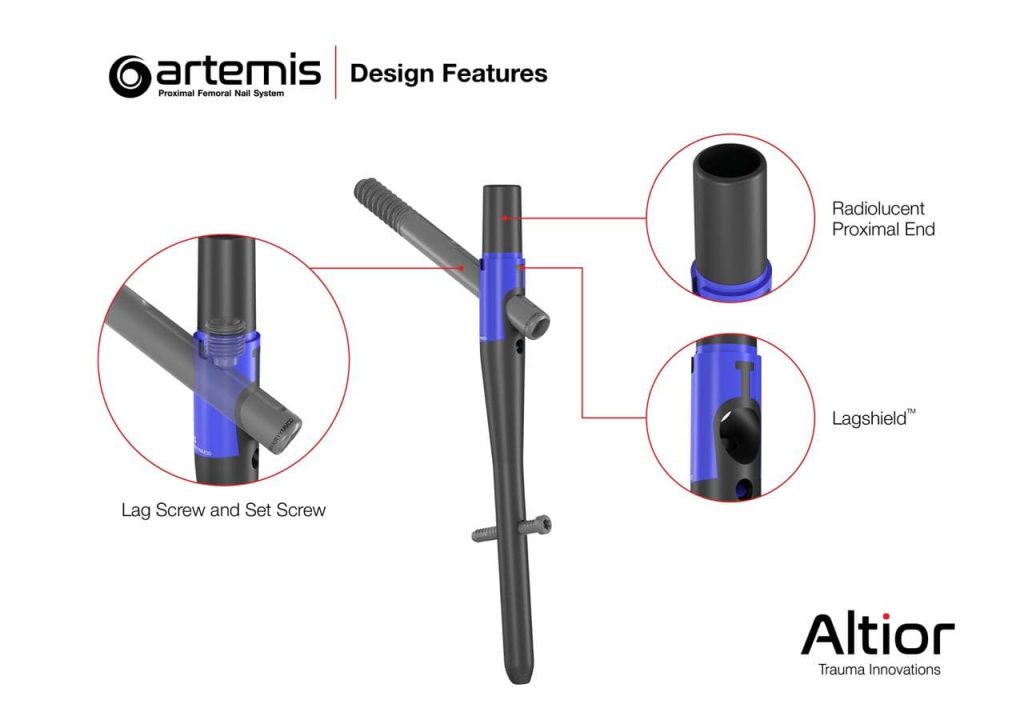
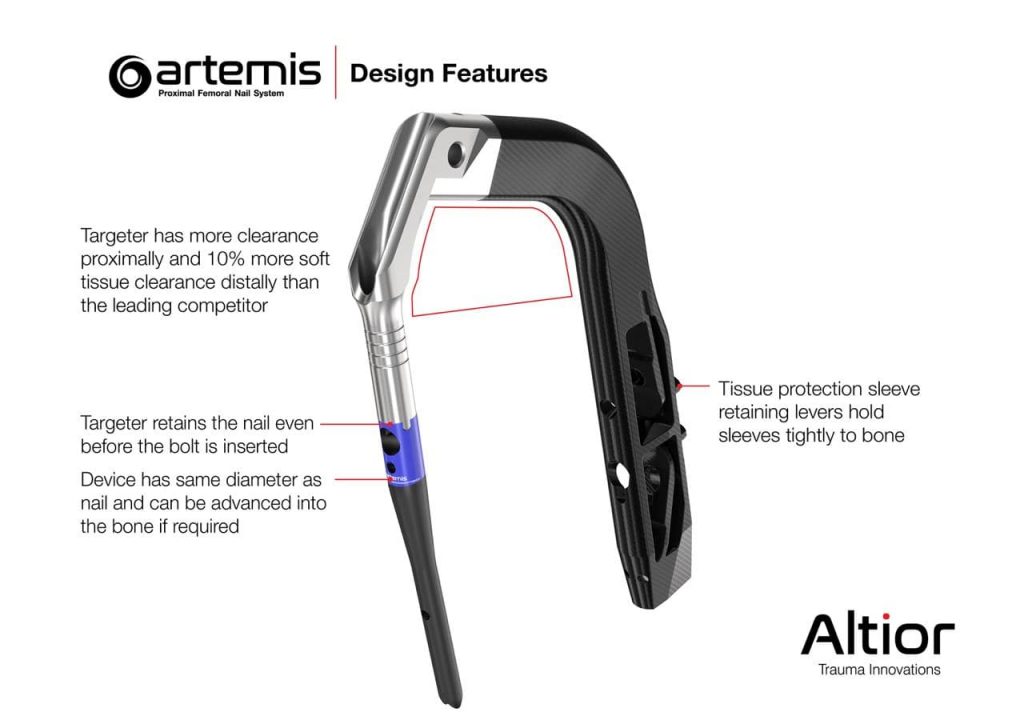
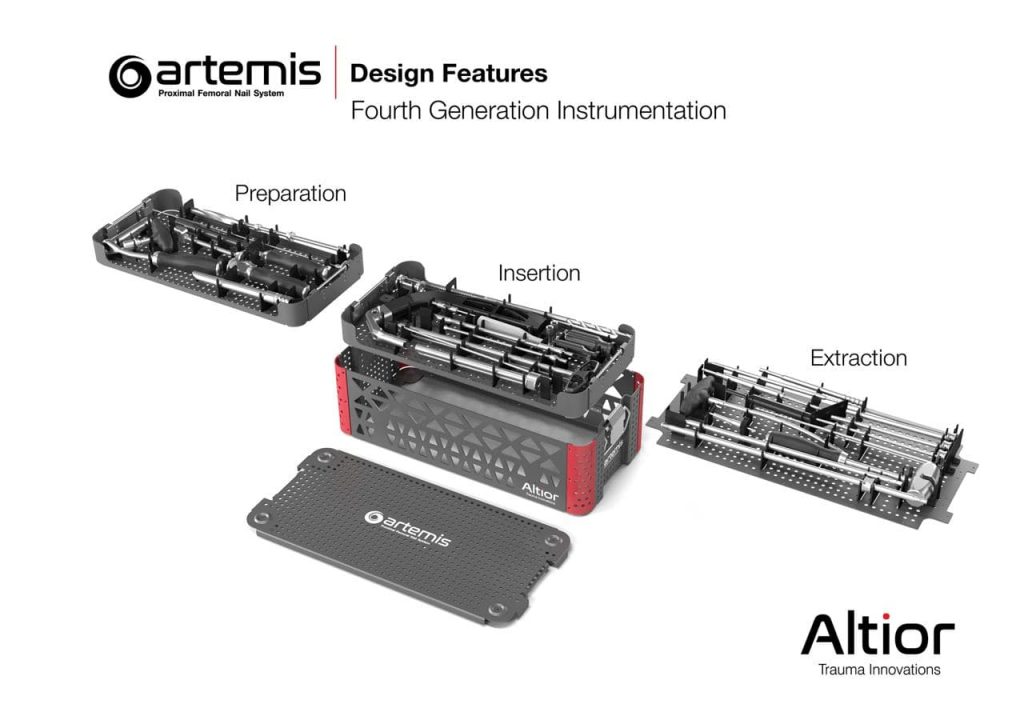
The Artemis System provides a fourth-generation nail with streamlined surgical instruments that allow for simplified implantation and superior patient benefits. Among other unique design features, the instrument set includes a novel anti-rotation pin that is designed to prevent head and neck rotation during reaming and implantation of the lag screw.
Dr. Walter W. Virkus, Director of Orthopaedic Trauma at Indiana University Health stated, “There are a number of features that make the Artemis nail better for our patients. The feature, I believe, that makes the biggest difference is the Lagshield™ which prevents titanium notching during lag screw reaming, thus reducing the potential for iatrogenic fracture of the nail prior to fracture healing.”
Of the $785M U.S. intramedullary nail market, cephalomedullary nailing accounts for 60%, or 160,000 annual procedures, of hip fracture surgeries. Altior Trauma plans to make a significant impact on this space and is targeting the first quarter of 2021 to begin marketing and selling the Artemis System.
ABOUT ALTIOR TRAUMA, A GLW, INC COMPANY
Altior Trauma is a privately held company, focused on the design and development of cost-effective implants for hip fractures. Their products are represented in the U.S. market through an exclusive distribution agreement with Innov8ortho, LLC.
Vadim Gurevich
Altior Trauma
+1 201-731-3103
contact@glwmed.com
Visit us on social media:
LinkedIn

 Tiger Buford – retained recruiter dissecting orthopedics
Tiger Buford – retained recruiter dissecting orthopedics