
The untold story of Carevature Medical
Carevature Medical is an Israeli startup that is changing the game in spinal decompression. It hasn’t been a straight road… it almost never is. But it HAS been one that has made its way through the typical landmines, funding difficulties, and commercial challenges that are part of every startup effort.
Yosi Weitzman was led to becoming an engineer in large part because he liked to solve problems with his own creations. After earning his Masters in Mechanical Engineering, Yosi began applying this curiosity to his professional work. He spent some time in the cardiovascular space working on minimally disruptive solutions before deciding to focus upon spine surgery, where less disruptive surgical techniques were becoming recognized as a key element in improving patient outcomes.
The Unmet Need is Discovered
Yosi recognized that the spinal industry had seen several technological advancements during the first decade of the 2000s, and that these new ideas were primarily focused upon the stabilization aspect of spine surgery. New expandable implants, advanced biologics, and navigation and robotics tools were beginning to proliferate in the space. However, Yosi observed that very little attention had been directed to the neural decompression aspect of a spinal procedure. Spine surgeons were using the same tools for spinal decompression (Kerrison Rongeurs, High-Speed Drills, etc.) that they had been using for decades!
Carevature Medical LTD was formed with a central idea that improving outcomes in spinal surgery can be best influenced by improving the technology and techniques that are used to perform the decompression itself. After all, neural compression and the resulting pain, numbness, weakness, etc. is typically the reason the patient shows up in clinic in the first place.
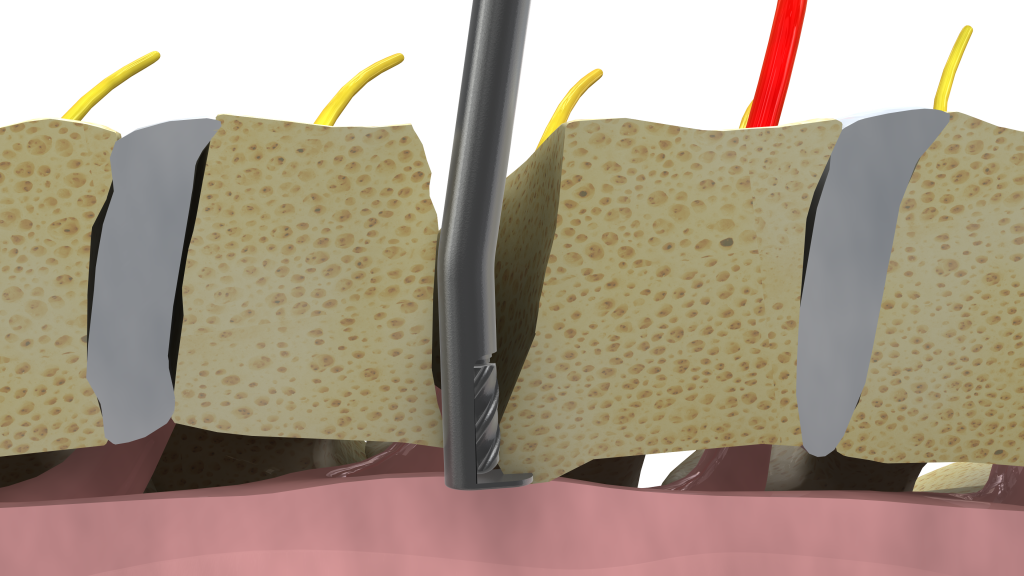
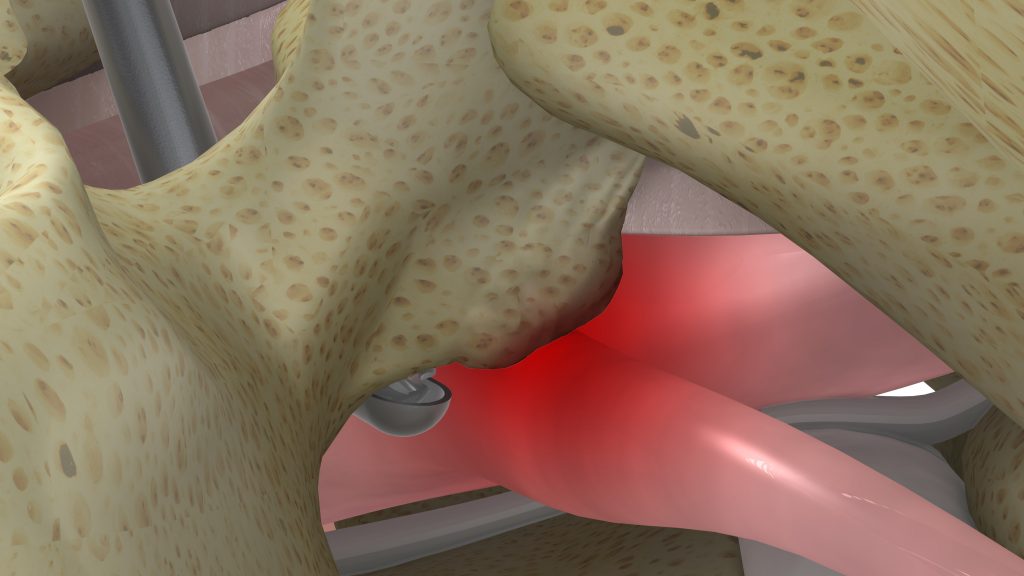
The early stakeholders who were working in Yosi’s garage observed that reaching and removing compressive pathology during spine surgery is challenging and potentially dangerous, due to the fact that the compression is often “around a corner” and outside the line of sight of the surgeon. Furthermore, the compressed neural structure is at risk for injury or dural tear with standard tools such as Kerrison Ronguers or High-Speed Drills.
They envisioned what appeared to be an easy solution: a shielded high-speed drill with a curve near the tip, allowing the surgeon to reach around the corner and remove the bony compression while protecting the adjacent nerve and preserving nearby healthy, structural bone. Why hadn’t anyone else thought of this, they asked themselves? Their early concepts made several leaps into the future… these concepts were designed for percutaneous application and included flexible cutting shafts. It was time to find a clinician partner for the venture, and Yosi began to call upon several spine surgeons across Israel for their thoughts.
The common reaction was, “what a great idea!” There was little to no criticism about the design principles, but the team was looking for someone with some healthy skepticism, who would share the unvarnished truth. They found this in a visit with Ely Ashkenazi, MD, a neurosurgeon at Assuta Medical Center in Tel Aviv. Dr. Ashkenazi told them, “you guys don’t understand spine surgery. This device is too futuristic and is not usable in its present form.” He went on to describe the changes that would need to be made to be compatible with current open, mini-open, and MIS techniques. “Come back to me when you have made these changes and I’ll not only use the product but will join you in the project.”
Several months later, Yosi was back with an updated design, and he and Ely formed Carevature Medical, LTD. They learned quickly that as with any “good idea”, many people HAD been thinking about the need for a “curved drill”. Even today, this is one of the initial reactions a surgeon will have when seeing the Dreal® (pronounced Dee-Ree-AL), for the first time… “I have been thinking about the need for a high-speed drill with a curve at the end since I first entered practice!” Or something along these lines.
The Big Challenge
Here is where the story takes a turn, and the answer to the question, “why was there no curved, high-speed drill on the market?” became more clear… While the idea SEEMS simple, it turns out that achieving 40-60,000 RPM (the speed necessary to efficiently cut bone) around a curved shaft is VERY challenging from an engineering point of view. The initial prototypes failed, and so did the subsequent attempts. Intense heat and mechanical stress and strain led to several different modes of failure, and days became months, and over a year passed. Yosi now could understand why there was no working product on the market even though “it was a simple idea that had been thought about by many.”
The Breakthrough
Since engineers are problem solvers, and since Yosi and Ely aren’t the type to give up very easily, they continued to work on development of a proprietary technology that would deliver the necessary RPM without overheating or failing. After much thought and internal deliberation, a unique design approach was applied, which finally led to the breakthrough… a curved, shielded, high-speed bone-cutting device that could rotate at 60,000 RPM, orders of magnitude higher than joint cartilage shavers that can reach only 8-10,000 RPM. They had met their initial goal.


Following initial cases in Israel and building enthusiasm for the opportunity to greatly improve the decompression landscape, Yosi recruited Bob Cook, a spinal industry veteran to lead the US sales effort. Bob quickly established traction in the US market with a group of spinal surgeons that immediately recognized the benefits of this breakthrough technology. A bayoneted design was added for surgeons that perform tubular micro-decompressions and the platform is now available for open, mini-open, and MIS approaches.
The path to bring this initial design to market involved all the typical elements that are necessary to commercialize a new idea. Access to funding, regulatory clearance, proof of commercial concept, intense learning, etc. Today, all of these checkpoints have been met. Carevature Medical is active in several markets, including the US, Latin and South America, and Israel. The CE Mark is pending and over 1500 commercial cases have been successfully performed throughout the world.
Spine surgeon adopters are using the technology regularly to perform decompression in uninstrumented cases, as part of a fusion or arthroplasty procedure, and throughout all regions of the spine. Several models are available for applications including foraminotomy, cervical and lumbar osteophyte removal, and intervertebral endplate preparation.
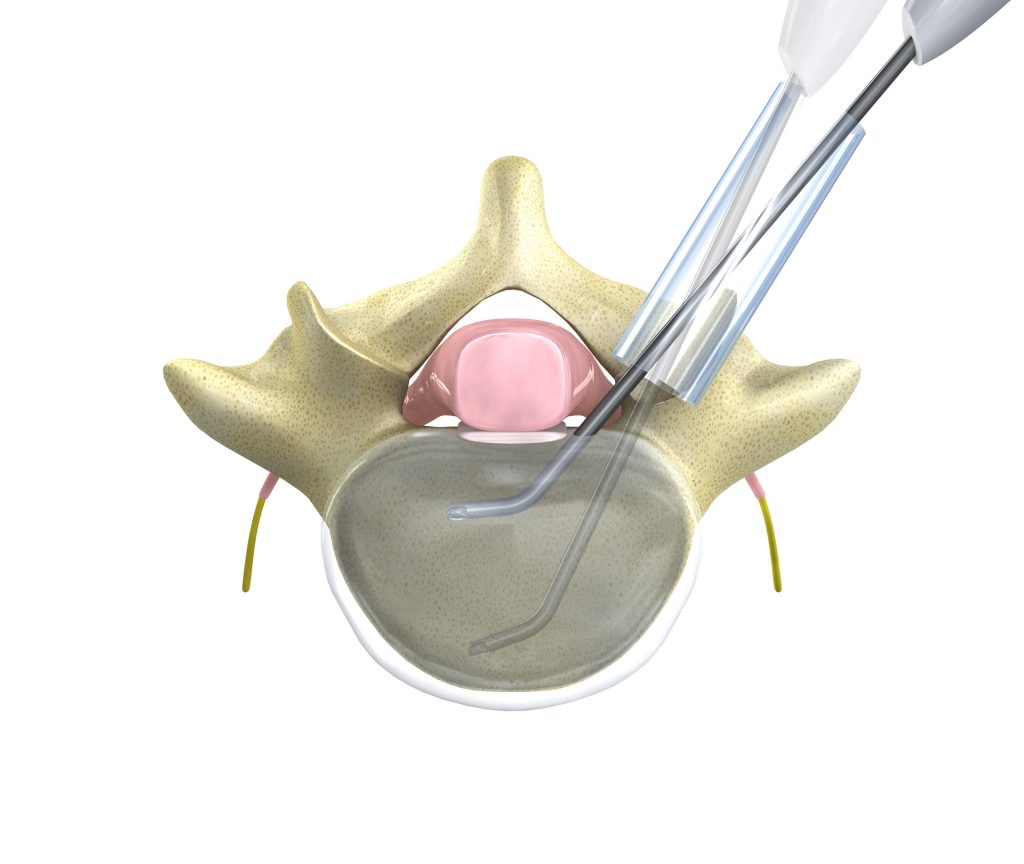
Early Adopter Physician Comments
Larry Khoo, MD: “Surgeons are still using the same tools for decompression that they have been using for decades… Carevature changes this with a decompression platform that offers advantages to surgeons and patients alike.”
John Peloza, MD: “The Dreal® system enables shorter and safer decompressions, preserving maximum healthy tissue and facilitating patient recovery… This system is intuitive, it’s easy to use and has become a valuable work tool for me, in both lumbar and cervical cases using a minimally-invasive approach.”
Scott Kutz, MD: “I have found the Dreal® to be useful for foraminotomies, ACDF, and TLIF procedures. The bayonetted version works particularly well through a tubular retractor. This technology allows me to perform better decompressions with lower risk for neural injury and spinal destabilization.”
Robert Eastlack, MD: “The Dreal® provides minimally invasive options that aren’t available with both traditional and other modern instruments alone allowing me to effectively target the impinging bone causing pain, and to preserve the soft tissue and bone critical to the patient’s spinal stability. This technology also has significant potential to prevent more extensive surgery.”
After the typical road bumps and obstacles that characterize many startup ventures, these are exciting times for Carevature Medical.
The company is expanding into new markets into the US, is growing rapidly and is working on the next round of investment to further scale the business.
For more information about product availability, distribution, or investment please reach out to Dennis Farrell, President of Carevature Medical, Inc. at 410-336-6651 or dennis@carevature.com.
website – https://www.carevature.com/

 Tiger Buford – retained recruiter dissecting orthopedics for you
Tiger Buford – retained recruiter dissecting orthopedics for you 