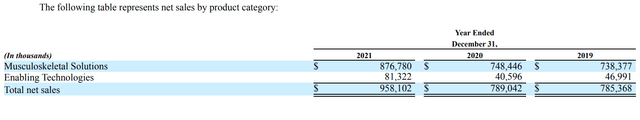Snapshot: I see 4 BIG RISKS with the Globus + Nuvasive merger
1/ Combining different cultures.
2/ Sales & distribution integration.
3/ CE Compliance at Globus.
4/ $1B debt at Nuvasive (Globus inherits NUVA’s poor financial health).
Globus Medical: Does The NuVasive Merger Make Sense? (Seeking Alpha)
Summary
- Globus Medical announced a merger with NuVasive on 8th February.
- The merger will create the second-largest spine business behind Medtronic, with 21% of the global market share.
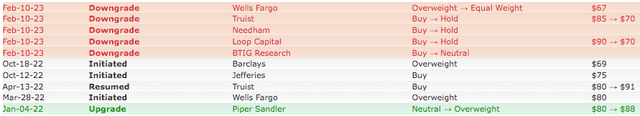
- Analysts expressed doubts about the potential benefits of this merger, lowered their Buy ratings to Hold, and slashed price targets.
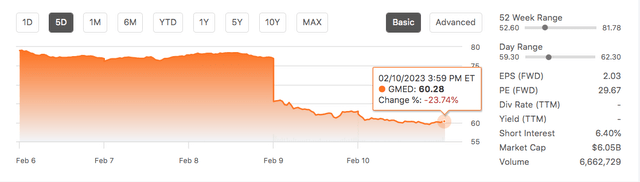
- Institutional investors fled these stocks. GMED share price fell 21.9% and NUVA, while rising briefly, ended the week 3% lower than Wednesday.
- Should retail investors of GMED and NUVA buy, hold, or sell their shares?
Preamble
Medical device companies fascinate me. Generally speaking, the business model of these companies is focused on meeting specific physical and medical needs. When these are met, thanks to humanity’s technological advancements, the quality of millions of lives is improved. It just feels good to be a shareholder in companies with meaningful missions.
There are several companies in this industry that I follow, such as Johnson & Johnson (NYSE: JNJ), Medtronic (NYSE: MDT), Intuitive Surgical (NASDAQ: ISRG), and STAAR Surgical (NASDAQ: STAA). For some sense of the magnitude of the medical devices market share, let’s make some comparisons. In FY 2022 JNJ brought in more than $27 billion, MDT generated $31.6 billion, ISRG made $6.2 billion worth of sales, and the two companies in this article are small in comparison; Globus Medical (NYSE:GMED) total revenue ending December 2021 was just $0.958 billion and NuVasive (NASDAQ: NASDAQ:NUVA) was at $1.1 billion.
As Globus Medical had a sudden 21.9% crash in share price over the past week after announcing its merger with NuVasive, I am prioritizing the research for this company as GMED and NUVA shareholders may want to have some facts to make their hold, sell or buy more decision.
Let’s begin by understanding their respective businesses, the risks involved, their financials, and how shareholders of both companies can think about this merger. Understanding the business and risks will help us understand if a merger makes sense. Examining the financials will let us see if the combined entity will be stronger with better cash flows or weaker with more debt (think AT&T’s failed merger with Warner).
Globus Medical Business Overview
Globus Medical develops and commercializes healthcare solutions to improve the quality of life of patients with musculoskeletal disorders. GMED medical devices are purchased by hospitals, ambulatory surgery centers, and physicians.
GMED has over 220 product launches across 50 countries worldwide and claims to offer a “comprehensive portfolio of innovative and differentiated technologies that are used to treat a variety of musculoskeletal conditions” through minimally invasive surgery (MIS) techniques.
GMED separates its products into two major categories: Musculoskeletal Solutions and Enabling Technologies.
Musculoskeletal Solutions
These products consist primarily of implantable devices, biologics, accessories, and unique surgical instruments used in an expansive range of spinal, orthopedic, and neurosurgical procedures. Surgical treatments for musculoskeletal disorders can be instrumented, which includes the use of implants, or non-instrumented, which forego the use of hardware but may include biologics. GMED’s broad spectrum of spine products addresses the vast majority of conditions affecting the spine including degenerative conditions, deformity, tumors, and trauma.
With almost 20 years in this competitive market, GMED provides comprehensive solutions that facilitate both open and minimally invasive surgery techniques. This includes traditional fusion implants such as pedicle screw and rod systems, plating systems, intervertebral spacers, and corpectomy devices.
Enabling Technologies
The Enabling Technologies division is the new addition to the revenue stream in 2017. The products offered are advanced hardware and software systems, and related technologies, that are designed to enhance a surgeon’s capabilities and streamline surgical procedures by making them less invasive, more accurate, and more reproducible to improve patient care.
In the 2021 10K, The Enabling Technologies are described as comprising of
… imaging, navigation and robotics (“INR”) solutions for assisted surgery which are advanced computer-assisted intelligent systems designed to enhance a surgeon’s capabilities, and ultimately improve patient care and reduce radiation exposure for all involved, by streamlining surgical procedures to be safer, less invasive, and more accurate. The market for our Enabling Technologies in spine and orthopedic surgery is still in the infancy stage and consists primarily of imaging, navigation and robotic systems. In spine, a majority of these technologies are limited to surgical planning and assistance in implant placement for increased accuracy and time savings with less intraoperative radiation exposure to the patient and surgical staff. As our Enabling Technologies become more fully integrated with our Musculoskeletal Solutions, a continued rise in adoption is expected. Furthermore, we believe as new technologies such as augmented reality and artificial intelligence are introduced, Enabling Technologies have the potential to transform the way surgery is performed and most importantly, continue to improve patient outcomes.
The growth in that division was jumpstarted by the FDA approval of the ExcelsiusGPS robotics and navigation system, which at the time of approval in 2017 was “the only Robotic technology that combines robotics and navigation on a single platform and supports three different imaging modalities, Preop CT, Interop CT and Peroscopy“.
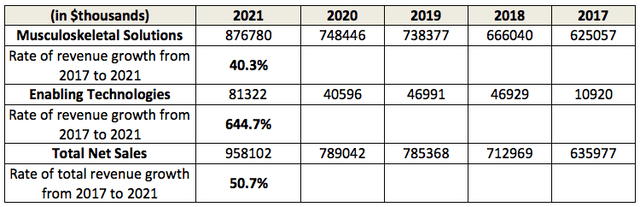
As can be seen from the table above, the revenue growth in this smaller division offers plenty of promise for GMED. The company does not break out the sales figures of the ExcelsiusGPS system or its unit price and remains coy about the revenue breakdown between 3D imaging and robots. To better understand the growth potential of this division, we can look at the unit price, system design, and related products.
According to this source, each ExcelsiusGPS system costs upwards of $1 million, more than Medtronic’s Mazor X system but less than the dominant player Intuitive Surgical’s Da Vinci system which cost a cool $2 million per system.
The ExcelsiusGPS platform was designed to be modular to serve as a foundation for add-ons functionality, and future clinical applications using artificial intelligence and augmented reality. Therefore, achieving growth in this division does not depend solely on the sale of the ExcelsiusGPS system. Ancillary products that value-add the system will continue to increase the revenue base.
For instance, through expandable technology like the ExcelsiusGPS Interbody Solutions, accessories such as the Globus Power High-Speed Drill, CREO MIS Posterior Stabilizer System, Rigid Robotic Arm, Navigated Disc Prep Instruments and more provide enhancements to the ExcelsiusGPS system, increase the use cases and deepen the stickiness of the system with surgeons.
Furthermore, in 2021 GMED expanded the use of ExcelsiusGPS from its focus on spinal surgeries to providing Cranial solutions. And in 2022, GMED started selling the ExcelsiusGPS Imaging systems.
The majority of the Enabling Technologies product contracts contain multiple performance obligations, including maintenance and support, and revenue is recognized as GMED fulfills each performance obligation, so with every completed surgery, parts like pedicle screws have to be repurchased, creating a consumption-based-like recurring revenue stream.
When the dominant Intuitive Surgical can still manage to almost double its revenue from $3.1 billion in 2017 to $5.7 billion in 2021, it speaks to the size of the total addressable market of the robotics surgery industry, and it explains why GMED’s management is able to increase the revenue in its Enabling Technologies division by 644% in the same period from 2017 to 2021. Granted, this Enabling Technologies division brought in only 9.2% of the total 2021 revenue. Yet, that is already 5 times the 1.7% of the total revenue it generated when it was first formed in 2017.
GMED’s 4 Growth Strategies
GMED has huge ambitions to become the market leader in the niche area of providing innovative solutions to promote healing in patients with musculoskeletal disorders. To accomplish this, the company lays out four strategies:
1. Research and Development: Launch new and innovative products every year. GMED currently has over 220 products; it launched 6 in 2021 alone. The company operates in an industry that is subject to rapid technological advancements so it must constantly improve existing products and introduce new products in order to continue to succeed.
2. Increase the size of the direct and distributor sales representatives in the U.S., to expand into new geographic territories and to deepen penetration in existing territories.
3. Continue to expand into international markets. As of December 31, 2021, GMED had an existing direct or distributor sales presence in 49 countries outside the United States.
4. Pursue strategic acquisitions and alliances.
On February 8, 2023, NuVasive entered into an agreement to merge with Globus Medical, and when news of this broke, GMED’s share price fall 21.9%. More on this in a while.
GMED has been growing through strategic acquisitions. Unlike its main competitor Medtronic whose $2493 million in R&D expenses in 2021 dwarfs GMED’s meager $97 million by 25 times, GMED cannot depend on its own R&D efforts to develop innovative products to beef up its pipeline. It has to make acquisitions to gain access to technologies developed by other companies that can be grafted onto its own products in accordance to the strategic direction of the management.
For instance, the following is a brief history of GMED’s acquisitions:
Besides acquiring adjacent technologies that can augment and advance GMED’s offerings, these also mean GMED expands its moat through patent protection. As of 31 December 2021, GMED has 1359 U.S. patents and 820 foreign patents. The merger with NuVasive will add another 730 U.S.-issued patents to this moat.
2 Risks Associated with GMED
Litigation
As in all medical procedures, especially those involving sensitive areas such as the spinal cord and the brain, product and procedural efficacy backed by years of rigorous studies and clinical data will help to convince hospitals and surgeons to adopt their products.
And that is a problem with GMED’s products. According to GMED’s 2021 10K (page 18),
… we currently lack the breadth of published long-term clinical data supporting the safety and efficacy of virtually all of our products and the benefits they offer that might have been generated in connection with other approval processes. For these reasons, surgeons may be slow to adopt our products, we may not have comparative data that our competitors have or are generating, and we may be subject to greater regulatory and product liability risks
Take for instance the case of Linda L. Boos, who filed a personal injury/products liability action against Globus Medical for injuries allegedly sustained from medical hardware that was allegedly manufactured and sold by them. The plaintiff asserts that on or around November 15, 2018, she underwent lumbar spinal surgery that required the implantation of surgical rods and screws into her lumbar spine, and these surgical screws and rods used in her surgery were designed, manufactured, assembled, marketed, distributed, and sold by Globus Medical. And on August 15, 2019, she was informed by her surgeon that her spinal implant had broken. The case is still ongoing as the judge denied GMED’s motion to dismiss.
Although my research did not show many litigations against GMED such that by Linda L. Boos regarding the efficacy of their products, a 2014 litigation (Bianco v. Globus Med., Inc.) illustrates the damage that litigations can have on a company’s bottom line. Then CFO Rick Barron said,
Net income for the year was $68.6 million, as compared to $73.8 million for the prior year. Net income for 2013 was impacted by $15.8 million provision for litigation. Fully diluted earnings per share for 2013 were $0.73 for the year compared to $0.80 for the prior year, while non-GAAP earnings per diluted share, which excludes the provisions for litigation was $0.90 in 2013, compared to $0.79 for 2012.
With the merger with NUVA, this also means any damages resulting from litigations (such as Kolar v. NuVasive) against NUVA will be borne by GMED.
CE Compliance
The next major concern I have is with the expiration of the CE mark. One of the risks highlighted in the 10K says:
The European Union/European Economic Area (“EEA”) requires a CE mark in order to market medical devices. Many other countries, such as Australia, India, New Zealand, Pakistan and Sri Lanka, accept CE or FDA clearance or approval. Other countries, such as Brazil, Canada and Japan, require separate regulatory filings.
Compliance with these requirements entitles us to affix the CE conformity mark to our medical devices, without which they cannot be commercialized in the EEA.
Following the expiration of the existing Directive 93/42 CE mark (May 2024), all medical device companies manufacturing and/or marketing products in the EEA, including Globus, will be required to comply with requirements of the Medical Devices Regulation EU 2017/745, which are generally stricter and include increasing technical documentation requirements and altering the classification of some products.
Most devices that are CE marked under Directive 93/42 may continue to be marketed in the EU under certain conditions until May 2024, at which point these products must comply with the new regulation. Products placed on the market under the Medical Device Directive may continue to be sold for one year after May 2024.
With the departure of the UK from the EU in January 2021, while CE marking will continue to be accepted by the UK until July 2023, a separate UKCA mark will be thereafter required to market a device in the UK.
That could signal a mid-term impact on the top-line revenue. Although the sales of GMED’s products in the EU can still continue until May 2025 (one year after expiration), it will mean more effort and money will need to be expended to get approval under the new and stricter standards. Even worse, the sale of GMED’s products in the UK has a shorter runway, till July of this year.
When these are taken together with the lack of clinical data to prove the efficacy of GMED’s products, I believe at least some of their products may face compliance issues and hence their continued sales may be delayed until compliance is met, and in the worst case scenario some products may be discontinued. That would not only affect sales volume but also the value of the assets GMED holds on hand.
As a supplier of medical equipment, GMED is required to hold high levels of inventory. According to the company,
Many of our Musculoskeletal Solutions products come in sets, which feature components in a variety of sizes to satisfy the particular patient’s anatomical needs. In order to market our Musculoskeletal Solutions products effectively, we often must maintain implant sets consisting of the full range of product sizes. For each surgery, fewer than all of the components of the set are used, and therefore certain portions of the set, like uncommon sizes, may become obsolete before they can be used. In the event that a substantial portion of our inventory becomes obsolete, it could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
And in the scenario when certain GMED products do not meet compliance and are not saleable, these will have to be written off.
Therefore it is positive that international sales only make up a smaller percentage of the total volume, so that mitigates the concern somewhat, but when part of the company’s growth strategy is to expand sales internationally, it means that management must ensure that they get the compliance they need in time so sales do not get interrupted.
And this is a good segway to NuVasive (NASDAQ: NUVA).
The merger between the two companies must make sense to the respective management teams. A study of NUVA’s business below will make a clearer case for or against the merger.
NuVasive Business Overview
In NUVA 2021 10K, the company claims to be:
… a global medical technology company focused on developing, manufacturing, selling and providing procedural solutions for spine surgery, with a guiding purpose to transform surgery, advance care and change lives. We offer a comprehensive portfolio of procedurally integrated spine surgery solutions, including surgical access instruments, spinal implants, fixation systems, biologics, and enabling technologies, as well as systems and services for intraoperative neuromonitoring. In addition, we develop and sell magnetically adjustable implant systems for spine and specialized orthopedic procedures. For the year ended December 31, 2021, we generated net sales of $1.1 billion, including sales in more than 50 countries.
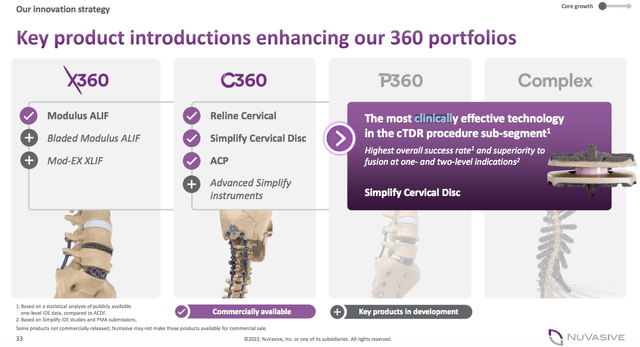
NUVA has two core products: Spine Hardware (X360 portfolio and C360 portfolio) and Specialised Orthopedics. The P360 is the next key product in development and received mentioned for the first time in the Q3 2022 earnings call.
The X360 is a comprehensive approach to lateral single-position surgery that leverages advanced techniques and technologies to deliver patient specific-care while enhancing operating room workflow and efficiency.
In 2020, NUVA launched the C360 system, a comprehensive portfolio to support anterior and posterior cervical spine surgery.
What should be highlighted from the X360 portfolio is the XLIF (Extreme Lateral Interbody Fusion). It is the only lateral approach procedure proven by over 10 years of clinical evidence with more than 400 published clinical studies supporting the procedure, documenting positive clinical outcomes such as reduced blood loss, less time in the O.R., and shortened hospital stays as compared to traditional posterior fusion procedures. This product is precisely the kind that can help to address GMED’s lack of products that can boast of the same clinically supported efficacy.
I will highlight the following NUVA products that align well with GMED’s products: the Pulse, Advanced Materials Science Portfolio, MAGEC, and Simplify.
The Pulse platform is a software ecosystem that “integrates multiple hardware technologies into a single, condensed footprint in the operating room, including radiation reduction, imaging enhancement, rod bending, navigation, IONM, and spinal alignment tools” which complements well with GMED’s ExcelsiusGPS robotics and navigation system.
NUVA’s Advanced Materials Science portfolio of specialized spinal implants is designed to advance spinal fusion by enhancing the osseointegration and biomechanical properties of implant materials, and this portfolio fits well within GMED’s Spine Solutions.
NUVA’s MAGEC technology forms the basis for its preCICE line of products, designed to support complex orthopedic reconstruction, such as trauma and limb length discrepancy, and this augments GMED’s earlier acquisition of Synoste Oy, a Finnish engineering company that specializes in the research and development of a limb lengthening system.
In 2021, NUVA purchased Simplify Medical for $150 million to acquire its motion-preserving cervical artificial disc technology for cTDR procedures. Simplify’s Cervical Disc is the only cTDR to achieve clinical superiority at primary endpoints for 1- and 2-levels compared to anterior cervical discectomy and fusion (ACDF). This product will complement GMED’s SECURE®-C cervical disc.
Simplify’s Cervical Disc product is already contributing to NUVA’s sales. In Q3 2022, NUVA CFO Matt Harbaugh shared,
U.S. Spinal Hardware net sales were $163.5 million, representing a 12.7% increase over the prior year period. Our cervical portfolio achieved a record quarter in net sales led by the C360 portfolio and the Simplify Cervical Disc, which continues to experience strong surgeon demand due to its differentiated features.
A Merger That Analysts Dislike
News of a possible merger between GMED and NUVA first appeared in 2021. Back then, Piper Sandler analyst Matt O’Brien said,
We do believe NUVA is undervalued, the addition of Simplify would be welcome at GMED, and the management team at Globus would likely be able to pull a ton of costs of the combined entity, but, at the end of the day, we simply do not see it happening,” O’Brien wrote.
When news of the confirmed merger broke on 8 February 2023, and now that he has seen it, O’Brien was less positive. He wrote a note to his investors,
For GMED, we see a bunch of challenges to the deal, namely differing cultures, sales force and customer dislocation, and doubling down (essentially) in a slower growth category of orthopedics, which is the spine market.
He also dropped NuVasive from Buy to Neutral as he believes that could be antitrust concerns that can stop the deal from going through.
Other analysts followed suit in cutting their price targets and changing their stance from Buy to Hold.
Investors reacted as well. By the end of the week, the GMED stock price has fallen by 21.9% to close at $60.28 on 10 February 2023 while NUVA’s stock price dipped by 3%.
My Take on the Merger: It Is Not So Bad
Firstly, let’s examine what GMED pays for the merger.
This is an all-stock deal. NuVasive shareholders will receive 0.75 of a share of Globus Medical Class A common stock for each NUVA common stock they own at the close of the transaction, expected in mid-2023.
There are 51.9 million NUVA shares. If every share is converted to 0.75 GMED shares, that means GMED has to issue 51.9 x 0.75 = 38.925 million new shares for the NUVA shareholders.
There are currently around 103 million GMED shares. Issuing another 38.925 million shares will increase the total share count to 141.925 million. This share dilution translates to GMED shareholders owning 72.6% of the original company. GMED shareholders do not see dilution as a positive as adding more shareholders means their ownership of the company is reduced.
However, if GMED can buy over a company with no cash, that could be a good thing if it gets more than what it pays for. This deal is valued at $3.1 billion, valuing NuVasive’s shares at a 26% premium to its closing price on 8 February.
However, based on GMED closing price of $60 on 10 February, that is equivalent to paying around $60 x 38.925 million shares = $2.335 billion for NUVA. NUVA’s market capitalization on 10 February 2023 is $2.32 billion, so I would say that GMED is not overpaying for this merger with NUVA at that price.
Next, let’s examine what GMED gets for the merger.
A merger is like marriage. One gets the good, the bad, and the ugly.
Let’s start with the Good.
I have already mentioned some of the positives above regarding GMED getting NUVA’s technologies, patents, products, revenue streams, and of course NUVA’s sales representatives which are instrumental in bringing in sales.
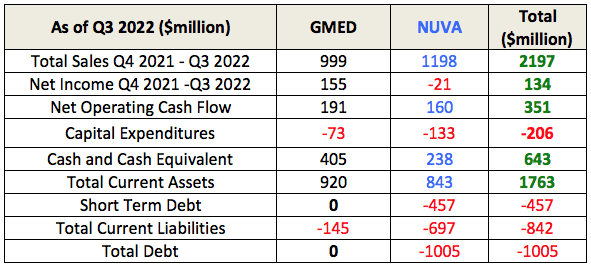
GMED’s is clearly in a better financial state than NUVA as of Q3 2022. It has a positive net income that fully covers its current liabilities. It is debt free and has $405 million in cash and cash equivalent.
After the merger (if approved by SEC), it will get NUVA’s $238 million in cash and cash equivalents, $1.1 billion in sales (which has remained around $1 billion for each of the past 5 years), an additional $160 million in operating cash flow, and the previously mentioned technologies, patents, and sales representatives.
The combined entity will become the second-largest spine business behind Medtronic with 21% of the global market share.
Next, the Bad.
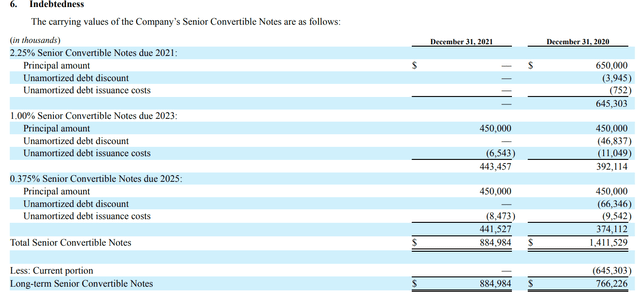
GMED will inherit NUVA’s debts, totaling $1 billion of which $450 million Senior Convertible Notes are due in 2023. This is manageable as the combined entity will have $645 million in cash and cash equivalent. The next payment is only due in 2025. This is borrowed at a fixed rate of 0.375% and due only 3 years later. Even assuming no growth in operating income, two years of combined operating income is more than sufficient to pay off this convertible bond, so this is also not a major concern.
Finally, the Ugly.
GMED has purchased an inferior business that it has to improve.
GMED is a business that has generated positive net income from 2009 to 2021. Its net income increased from $49 million in 2009 to $149 in 2021. From 2017 to 2021, GMED increased sales by 50%. An investor holding GMED stock from 2013 to 2023 would have seen an annual rate of return of 18.3% (even after the 21.9% drop over the past week) versus the S&P 500’s 11.94%.
NUVA however has generated 4 years (2021, 2020, 2014, and 2011) of negative net income in the same 13-year period. An investor who held NUVA shares from January 2013 to February 2023 would have an annual rate of return of just 10.42%.
No wonder NUVA shareholders initially cheered and their stock price went up upon hearing the news, while GMED shareholders dumped the shares.
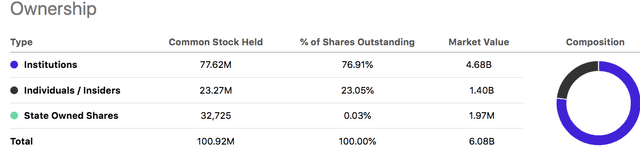
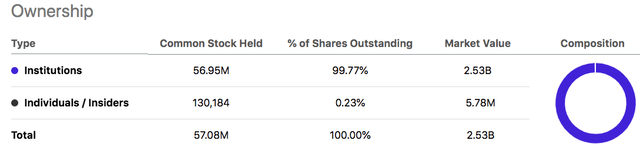
However, most of GMED’s and NUVA’s shares are owned by institutional investors and not retail. Analysts are skeptical of this merger and do not approve.
BTIG’s Ryan Zimmerman wrote in a note to investors, “While we sound skeptical as we write this, we acknowledge that there are clear benefits to scale in the Spine market. Closing the gap, from a profitability perspective … will be a challenge.”
JPMorgan (JPM) analysts Allen Gong and Robbie Marcus had this to say to their investors, “Bigger can be better, but a history of challenged ortho consolidation keeps us cautious about the deal.”
RBC’s Singh called the acquisition “somewhat of a surprise to us, given traditional differences between the two firms, and the history of high transaction dis-synergies (up to 10% of sales),” when spinal firms combine.
Once their institutional investors get the memo, they dumped shares of both companies, evident from the huge selling volume in the two days after the merger announcement was made.
Valuation
Given a choice between the two companies, I will only consider investing in GMED over NUVA because it is the better company by virtue of its consistent track record of growing revenue and net income. Plus the fact that its price dropped by 21.9% in the past week suggests it could be trading in value territory.
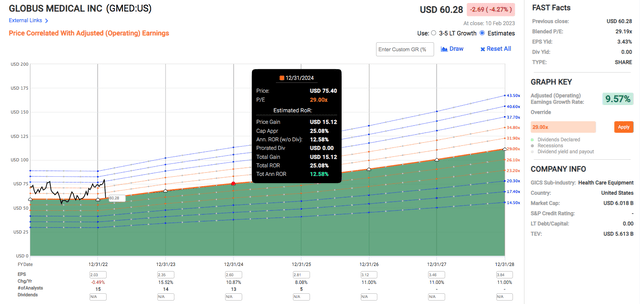
Globus Medical has been trading at an elevated normal P/E of 34.6 for the period between 2018 to 2023. It reached a low of 22.8 during the depths of the pandemic in March 2020 before peaking at 46.55 in July 2021 when the stock reached an all-time high of $83.69. GMED’s adjusted earnings CAGR has been consistently around 11%.
I doubt the PE will sink as low as 22.8 again with the majority of the world having acquired herd immunity and opened up, including hospitals. Still, for a bear case scenario, I will consider GMED trading at a P/E of 23.
Assuming that GMED trades at a PE of 29, which is below its past 5-year normal P/E of 34.6, and grows earnings at a rate of 9.57%, it could potentially provide a total annualized rate of return of 12.58% after two years. If the stock trades at a P/E of 33 which is near its historical normal of 34.6, the total annualized rate of return rises to 20.55%.
If it trades down to a P/E of 23.2 near the bear case, the stock will stay flat. From the perspective of P/E, the risk-reward consideration seems favorable, with 0.04% in the worst case, 12.58% in the normal case, and 20.55% in the best case.
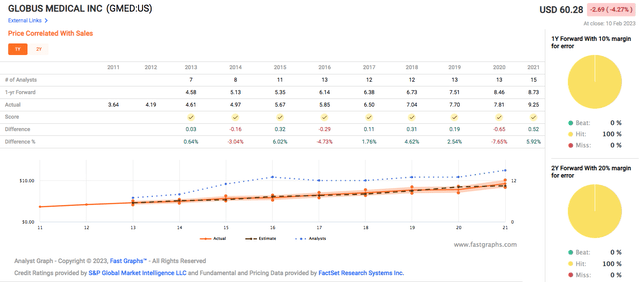
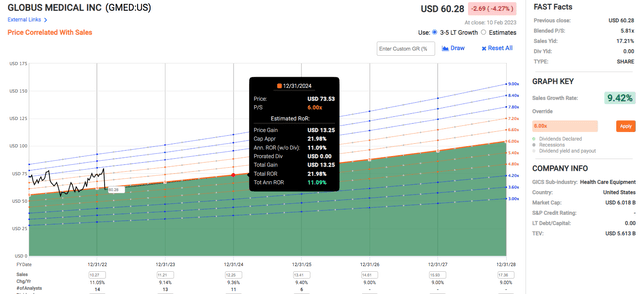
From a P/S point of view, GMED’s sales CAGR has been consistently around 9%. GMED has met analysts’ sales expectations 100% of the time for both the 1-year and 2-year forecasts.
Its normal P/S has been around 7.25 for the past 5 years, so assuming a lower P/S of 6 and a sales growth rate of 9.42%, GMED could provide a total annualized rate of return of 11.09% after two years. If the stock trades up to a P/S of 7.2, the total annualized rate of return doubles to 22.34%.
If GMED trades down to a P/S of 4.8 (it was at a P/S of 4.82 in March 2020), it could return a total annualized rate of return of -1.29%. Based on this range of estimates, the risk-reward for GMED is quite favorable, with -1.29% in the worst case, 11.09% in the normal case, and 22.34% in the best case.
Of course, the above valuation projections are based on just one company – Globus Medical. And with the uncertainty surrounding the completion of the proposed merger, I will sit this one out even if the valuation and risk-reward for GMED look favorable.
Conclusion
Interested parties will be keeping a watchful ear on 21 and 22 February 2023 as Globus Medical and NuVasive will respectively report full financial results for 2022 and provide their financial outlook for 2023 during their earnings announcement.
Meanwhile, let’s discuss scenarios. My take will be a HOLD, for both GMED and NUVA shareholders, and I give the reasons in the table below.
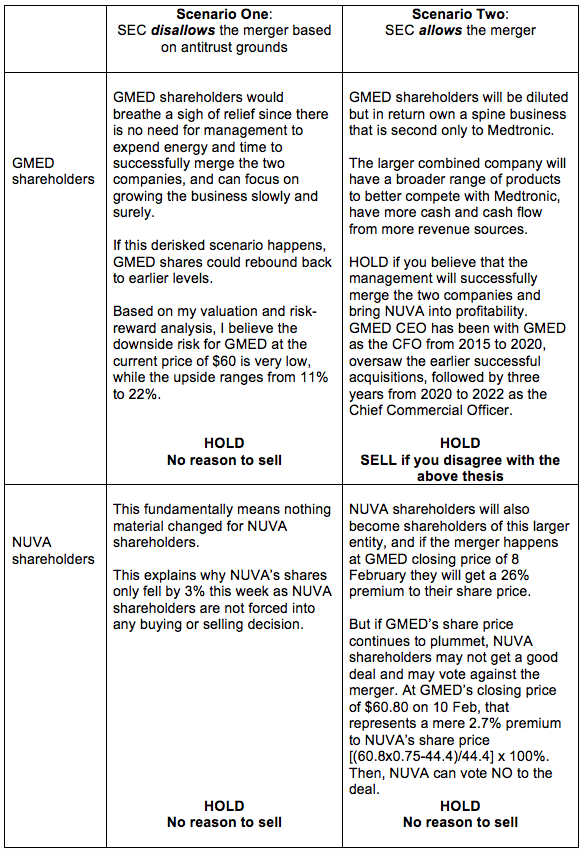
The only SELL scenario is if a GMED shareholder does not have confidence in GMED’s management to successfully merge the two companies into an efficient, working entity capable of taking on the likes of Medtronic and Intuitive Surgical.
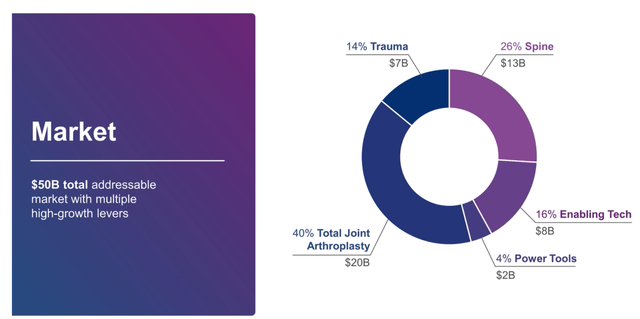
I believe that GMED is a good company, with good prospects, is serving in an area of critical need in healthcare, and has a rapidly growing Enabling Technologies division. With GMED insiders owning almost one-quarter of the shares outstanding, I have no doubt that the interests of the GMED management are aligned with shareholders. If the merger works, the combined company will have well-defined business segments with specialized products to target a $50 billion total addressable market.
If it were a standalone company, I would consider initiating a position in it. But even as a standalone company, GMED is handling some concerns such as meeting the new CE compliance and handling litigations. However, as GMED is considering merging with NUVA, a company that requires help to get back to profitability, that will take up even more of management’s attention from attending to the more important, strategic aspects of the company’s growth, to managing the operational issues of a merger, like resolving cultural differences, standardizing compensation structure for the sales representatives from both companies, cutting costs to improve profitability, and cumulatively these multiple concerns add up for me.
The two firms expect their combined salesforce and shared operational strengths will better fend off competition.
Globus Medical said it has agreed to acquire NuVasive in an all-stock deal valued at $3.1 billion and aimed at creating the world’s “leading musculoskeletal technology company.”
Audubon, Penn.-based Globus makes products for spinal surgery, imaging, joint reconstruction and trauma, and San Diego-based NuVasive specializes in products for spine surgery.
With the move, the companies said they expect to benefit from a larger combined sales presence and each other’s operational strengths, such as Globus’ in-house manufacturing capacity and NuVasive’s global distribution networks. The combined firm may reduce costs by as much as $170 million over three years, Globus said in a statement on Thursday.
Combined, the company will be the second-largest spine business behind Medtronic, and will have about 21% of the global market share, RBC Capital Markets analyst Shagun Singh wrote in a research note on Thursday.
The deal values NuVasive’s shares at a 26% premium to their price at Wednesday’s close of trading.
Skepticism and caution
Analysts expressed some concerns about the potential benefits of the transaction.
“We didn’t believe that [Globus] would want to combine with [NuVasive], as both companies have a different approach to commercialization, profitability, and corporate culture,” BTIG’s Ryan Zimmerman wrote in a note to investors after the announcement. “While we sound skeptical as we write this, we acknowledge that there are clear benefits to scale in the Spine market,”
“Closing the gap, from a profitability perspective … will be a challenge,” he added.
For JP Morgan analysts Allen Gong and Robbie Marcus, “bigger can be better, but a history of challenged ortho consolidation keeps us cautious” about the deal, they wrote in a note to investors.
RBC’s Singh called the acquisition “somewhat of a surprise to us,” given traditional differences between the two firms, and the history of “high transaction dis-synergies (up to 10% of sales),” when spinal firms combine.
Still, “we believe the deal could make sense given minimal portfolio overlap, as well as ability to drive global scale and operational synergies,” Singh added.
Shares fall
Some investors signaled they may share the same concerns. Shares of Globus declined about 20 percent in midday trading on Thursday, while NuVasive stock rose 1.6%. Shares of both firms have fallen about 6% in the past 12 months.
Globus CEO Dan Scavilla said the acquisition will help the company become “the leading musculoskeletal technology company in the world.”
The combined company will have a presence in more than 50 countries with more than 5,000 employees.